![Ni(H2O)6] 2+ (aq) is green in colour whereas [Ni(H2O)4 (en)]2+(aq)is blue in colour, give reason - YouTube Ni(H2O)6] 2+ (aq) is green in colour whereas [Ni(H2O)4 (en)]2+(aq)is blue in colour, give reason - YouTube](https://i.ytimg.com/vi/pONeuFEpHnU/maxresdefault.jpg)
Ni(H2O)6] 2+ (aq) is green in colour whereas [Ni(H2O)4 (en)]2+(aq)is blue in colour, give reason - YouTube
What is the hybridization, transition, spin, colour, magnetism, and geometry of [Ni(H2O) 4] +2? - Quora
![Aqueous solution of Ni^2 + contains [Ni(H2O)6]^2 + and its magnetic moment is 2.83 BM . When ammonia is added in it, comment on the magnetic moment of solution. Aqueous solution of Ni^2 + contains [Ni(H2O)6]^2 + and its magnetic moment is 2.83 BM . When ammonia is added in it, comment on the magnetic moment of solution.](https://d1hj4to4g9ba46.cloudfront.net/questions/2015518_249889_ans_bbd2828107e04c46b63fdcb0f0c04801.jpg)
Aqueous solution of Ni^2 + contains [Ni(H2O)6]^2 + and its magnetic moment is 2.83 BM . When ammonia is added in it, comment on the magnetic moment of solution.
2·2Н2О and the complexes M(HTBA)2(H2O)2 (M = Ni, Co, Fe) - ScienceDirect Crystal structure and properties of the precursor [Ni(H2O)6](HTBA)2·2Н2О and the complexes M(HTBA)2(H2O)2 (M = Ni, Co, Fe) - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0277538713008310-fx1.jpg)
Crystal structure and properties of the precursor [Ni(H2O)6](HTBA)2·2Н2О and the complexes M(HTBA)2(H2O)2 (M = Ni, Co, Fe) - ScienceDirect
![SOLVED: The compound [Ni(H2O)6]Cl2 is paramagnetic. Determine the oxidation number of nickel in this compound, the most likely geometry of the coordination around the nickel, and the possible configurations of the d SOLVED: The compound [Ni(H2O)6]Cl2 is paramagnetic. Determine the oxidation number of nickel in this compound, the most likely geometry of the coordination around the nickel, and the possible configurations of the d](https://cdn.numerade.com/ask_previews/543ac424-a25c-42c6-88c8-ae8f1d1a07d2_large.jpg)
SOLVED: The compound [Ni(H2O)6]Cl2 is paramagnetic. Determine the oxidation number of nickel in this compound, the most likely geometry of the coordination around the nickel, and the possible configurations of the d
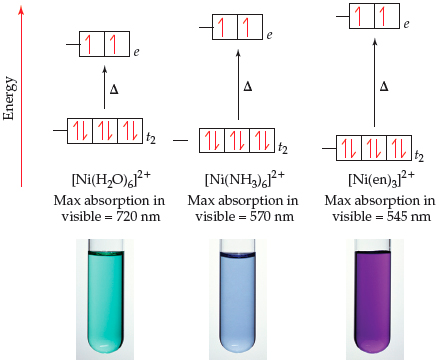
✓ Solved: Describe the distribution of d electrons in [Ni(H2O)6]^2+, using crystal field theory. How...

2 and [Ni(D2O)6](ClO4)2 at Various Temperatures | Semantic Scholar FIR Spectra of [Ni(H2O)6](ClO4)2 and [Ni(D2O)6](ClO4)2 at Various Temperatures | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/ecaf6b0fee69dc0a5060cdb8d7e5d0c7d395a06e/4-Table1-1.png)

![A solution of [Ni(H2O)6]^2 + is green, but a solution of [Ni(CN)4]^2 + is colorless. Why? A solution of [Ni(H2O)6]^2 + is green, but a solution of [Ni(CN)4]^2 + is colorless. Why?](https://d1hj4to4g9ba46.cloudfront.net/questions/1356080_1287162_ans_2cc4810034ce4d16bbf84081945e7afd.jpg)
![A solution of [Ni(H2O)6]^(+2) is green but a solution of [Ni(CN)4]^(-2) is colourless explain. A solution of [Ni(H2O)6]^(+2) is green but a solution of [Ni(CN)4]^(-2) is colourless explain.](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/647809885_web.png)
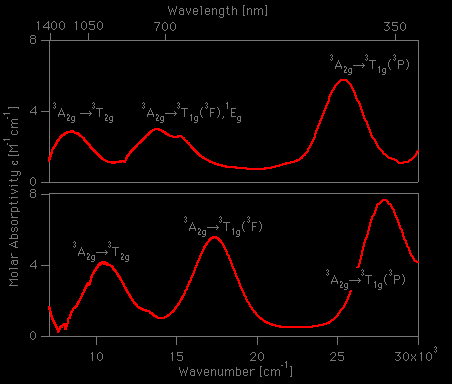
![a) Absorption spectrum of the [Ni(H2O)6]²⁺ complex in the spectral... | Download Scientific Diagram a) Absorption spectrum of the [Ni(H2O)6]²⁺ complex in the spectral... | Download Scientific Diagram](https://www.researchgate.net/profile/Petya-Petkova-7/publication/279288804/figure/fig1/AS:1132476902711296@1647014942438/a-Absorption-spectrum-of-the-NiH2O6-complex-in-the-spectral-region-395-795-nm_Q320.jpg)
![Absorption spectra of [Ni(H 2 O) 6 ] 2+ and [Ni(NH 3 ) 6 ] 2+ in... | Download Scientific Diagram Absorption spectra of [Ni(H 2 O) 6 ] 2+ and [Ni(NH 3 ) 6 ] 2+ in... | Download Scientific Diagram](https://www.researchgate.net/publication/228364596/figure/fig3/AS:667854408003587@1536240307249/Absorption-spectra-of-NiH-2-O-6-2-and-NiNH-3-6-2-in-aqueous-solution-The.png)
![Solved 20. When H2O in the [Ni(H2O)6]2+ complex ion is | Chegg.com Solved 20. When H2O in the [Ni(H2O)6]2+ complex ion is | Chegg.com](https://media.cheggcdn.com/media%2F782%2F7824ed9d-14a5-4257-bc8c-05dc5a9cf139%2FphpvCnudZ.png)